Science
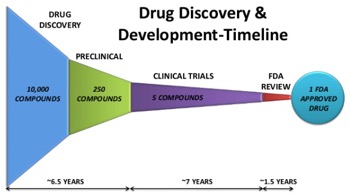
The pharmaceutical development process is complex, calls for significant investments in financial and human capital, and involves risks in project execution, the regulatory process, and scientific technical attrition. Further, the cost to gain market approval for a single product is estimated at over $900 million. Of the companies that set out to develop a drug beyond proof of principle, most fail due to lack of product efficacy or safety, or insufficient cash for clinical trials or business operations.
Industry data indicate a 20 percent likelihood for a compound to advance from initiation of phase 1 trials to market approval. With these odds, there’s a need to mitigate product development risks, from startup capital through early-and late-stage clinical trials. Further, leadership and commitment from the company’s executives are essential to determine tactics and strategies for developing product candidates, to set the right course for clinical development, and to manage capital. It calls for flawless operational execution, talented management, perseverance, and scientific expertise and vision.
Pre-Clinical
Pre-clinical analysis, gap assessment, project and program timelines, proposed next step experiments, experiment design, characterization of completed experiment, internal resourcing and outsourcing of work.
Clinical
Clinical stage analysis, gap assessment, project and program timelines, proposed next step clinical trials, study design, characterization of completed trial(s), internal resourcing and outsourcing of work.
Regulatory
Outline regulatory pathway in targeted regulatory jurisdiction. Bridge current clinical program to targeted regulatory requirement, project and program costs and timelines, proposed next step clinical trials, study design (requires statistical input), characterization of completed trials, internal resourcing and outsourcing of work, submission of CMC, preclinical and clinical data.
